|
Hydration
- Introduction
The
importance of the shape-function relationship in biomolecules is well
known. For proteins, nucleotides and other biomolecules to carry out their
tasks, they must have the correct three-dimensional conformation. Water,
with its large dipole moment and ability to act as both an excellent hydrogen
bond donor and acceptor, forms strong interactions with and dissolves
biomolecules which typically contain functional groups that are polar,
acidic, basic, hydrogen bond donors and/or hydrogen bond acceptors. As
a result, water plays an important roll in the conformation of biomolecules,
yet one that is not well understood at this time.
With
the recent development of soft ionization techniques such as matrix-assisted
laser desorption/ionization (MALDI) and electrospray ionization (ESI),
it has become possible to study large, fragile biomolecules in the gas
phase by mass spectrometric methods. This technique allows hydration effects
to be studied one water molecule at a time, providing a bridge between
the gas and solution phases.
In
an attempt to better understand the interaction between biomolecules and
water, we have performed temperature-dependent
equilibrium experiments on an electrospray
ionization mass spectrometer equipped with a drift cell to measure
ΔH° and ΔS° values for reactions of gas-phase protonated
and deprotonated peptides (P±zH)z± with water.
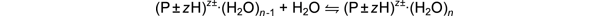
Molecular
mechanics (MM) and density functional theory (DFT) have also been
used to determine hydration energies as well as structures for the species
involved in the above reaction.
The following aspects of peptide hydration have
been summarized in reference 1:
- The significance
of ionic groups
- Hydration
of ionic groups
- The
ammonium group
- The
guanidinium group
- The
carboxylate group
- Molecules
with several ionic groups
- Multiply
charged ions
- Salt
bridges
- Structural
changes induced by hydration
- Change
of conformation
- Zwitterion
formation
- Entropy
of hydration
More
detailed information can be found in the following pages and in references
2 to 4:
References:
- "Hydration of Small Peptides" T. Wyttenbach, D. Liu, M.
T. Bowers Int.
J. Mass Spectrom. 2005, 240, 221-232
- "Hydration of Protonated Primary Amines: Effects of Intermolecular
and Intramolecular Hydrogen Bonds" D. Liu, T. Wyttenbach, M. T.
Bowers Int.
J. Mass Spectrom. 2004, 236, 81-90
- "Investigation of Noncovalent Interactions in Deprotonated Peptides:
Structural and Energetic Competition between Aggregation and Hydration"
D. Liu, T. Wyttenbach, C. J. Carpenter, M. T. Bowers J.
Am. Chem. Soc. 2004, 126, 3261-3270
- "Sequential Hydration of Small Protonated Peptides" D. Liu,
T. Wyttenbach, P. E. Barran, M. T. Bowers J.
Am. Chem. Soc. 2003, 125, 8458-8464
Bowers group members who have worked on these projects include:
|

