|
Hydration
- Competition with Peptide Self-Solvation
The
charge site on a peptide is stabilized by solvation, which can be accomplished
either by other portions of the peptide itself or by external solvents
such as water. The competition with self-solvation can, therefore, have
an effect on hydration. Temperature-dependent
equilibrium experiments and molecular
modeling reveal that peptides with a greater degree of self-solvation
have lower water binding energies.
|
|
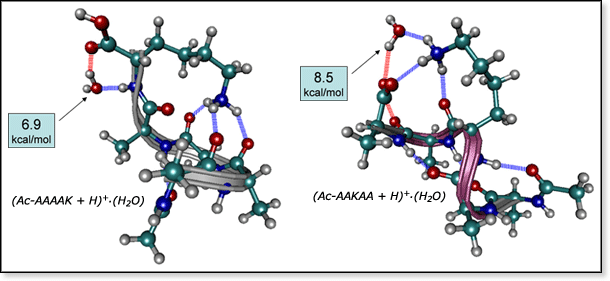
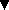 Molecular mechanics structures and experimental water binding energies
for
Molecular mechanics structures and experimental water binding energies
for
(Ac-AAAAK + H)+·(H2O) and (Ac-AAKAA + H)+·(H2O).
These two acetylated (Ac) pentapeptides differ only in the position of
the lysine residue (K), yet have different experimental water binding
energies. When lysine is at the N-terminus (structure on left), water
is bound 1.6 kcal/mol more weakly than when lysine is in the center position
(structure on right). Molecular mechanics studies show that this is due
to competition with peptide self-solvation. Lysine is the charge-carrying
group for both peptides, but when it is at the end of the peptide, it
is able to form three hydrogen bonds with the rest of the peptide. When
lysine is in the middle position, it can only form two intramolecular
hydrogen bonds. However, it can form a third hydrogen bond with water.
Because the water molecule in (Ac-AAKAA + H)+·(H2O)
is bound directly to the -NH3+ group on lysine it
forms a stronger bond than it does in (Ac-AAAAK + H)+·(H2O).
|