 |

|
|||
|
Hydration
- Solvation Shells & Charge Diffusion in Model Systems (Methylamine
& To
obtain a basic understanding of the interactions between peptides and
water, we have determined water binding energies and structures for the
model compounds |
|||
|
|
|||
|
|
|||
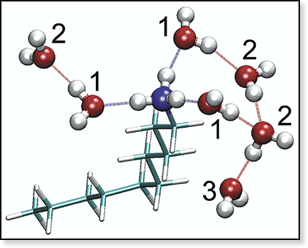 CH3(CH2)9NH3+·(H2O)7. The numerical labels indicate to which solvation shell the water molecules belong. This structure illustrates how the -NH3+ group controls the location of the water molecules. The seven waters form a cluster around the charge. |
|||