|
Hydration
- The Effect of the Peptide's Charge State
ESI
on peptides can produce ions with a distribution of charge states. For
instance, the 13-residue peptide neurotensin is formed in the +1, +2 and
+3 charge states under our experimental conditions. The charge state of
the peptide, not surprisingly, effects its hydration.
|
|
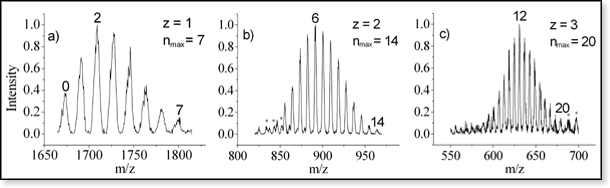
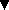 ESI mass spectra of (a) singly-, (b) doubly- and (c) triply-protonated
neurotensin recorded after exposure to 1.3 Torr of water vapor at 260
K. The labels above the peaks indicate the number of water molecules
coordinated to the peptide. At each charge state, the maximum number nmax
of waters adding to the peptide is roughly proportional to the number
of charges z on the peptide.
ESI mass spectra of (a) singly-, (b) doubly- and (c) triply-protonated
neurotensin recorded after exposure to 1.3 Torr of water vapor at 260
K. The labels above the peaks indicate the number of water molecules
coordinated to the peptide. At each charge state, the maximum number nmax
of waters adding to the peptide is roughly proportional to the number
of charges z on the peptide.
|
|
  Maximum number nmax of water molecules adding to the corresponding
peptide with charge state z at 1.3 Torr of water vapor and 260 K.
Mass spectra were measured for a variety of small peptides. In all cases,
the nmax/z ratio was found to be approximately 6. This result
indicates that the charge is the dominant factor in the hydration process
of these peptide ions and, without further evidence, seems to suggests
that the water molecules form a cluster around each charge on a peptide
as observed for n-decylamine.
Maximum number nmax of water molecules adding to the corresponding
peptide with charge state z at 1.3 Torr of water vapor and 260 K.
Mass spectra were measured for a variety of small peptides. In all cases,
the nmax/z ratio was found to be approximately 6. This result
indicates that the charge is the dominant factor in the hydration process
of these peptide ions and, without further evidence, seems to suggests
that the water molecules form a cluster around each charge on a peptide
as observed for n-decylamine.
 Molecular mechanics structures for bare and hydrated protonated dialanine.
Despite the apparently simple correlation between the number of charge
sites and the maximum number of water molecules adding to a peptide ion,
extensive MM analysis indicates the processes directing hydration of peptide
ions is complex. Unlike alkylamines, peptides have hydrophilic groups
other than the charge site. Not only do these other groups compete with
the charge sites for water, they compete with water to solvate the charge
sites.
Molecular mechanics structures for bare and hydrated protonated dialanine.
Despite the apparently simple correlation between the number of charge
sites and the maximum number of water molecules adding to a peptide ion,
extensive MM analysis indicates the processes directing hydration of peptide
ions is complex. Unlike alkylamines, peptides have hydrophilic groups
other than the charge site. Not only do these other groups compete with
the charge sites for water, they compete with water to solvate the charge
sites.
|