Lignin Deconstruction
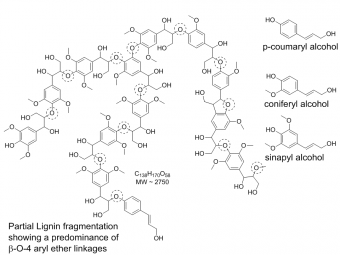
Lignin is the World's largest natural source of aromatic carbon. It is a complex polymer derived from p-coumaryl alcohol, coniferyl alcohol and sinapyl alcohol and coupled by aryl-ether bonds or other crosslinks. Fungal and bacterial microbes growing aerobically and anaerobically have evolved multiple enzymatic processes to break through the cellulosic components of lignocellulose, and release smaller aromatic breakout fragments. Aerobic fungal microbes degrade lignin, by secreting enzymes to break down lignocellulose in woody biomass. These enzymes include lignolytic peroxidases, such as manganese peroxidase (MnP), lignin peroxidase (LiP) and versatile peroxidase (VA) which are FeHeme enzymes; laccases which are multicopper oxidases; and aryl oxidases which produce H2O2 in the process of oxidizing primary aryl alcohols to aldehydes. The FeHeme peroxidases each produce a diffusible oxidant to attack lignin in the form of a Mn(III) carboxylate complex or an organic radical. Fe-heme and vanadium chloroperoxidases are other extracellular ascomycete enzymes from Caldariomyces fumago and Curvularia inaequalis, respectively. These enzymes produce the oxidant, HOCl, and also chlorinated compounds when the fungi are grown on woody biomass.
We have a two-pronged approach to lignin breakdown in the Butler lab, bio-mimetic oxidation and microbial disassembly. Looking for biological solutions to lignin degradation, we are interested in patterns of gene expression as a function of lignin source in a growth culture, with a particular focus on LiP, MnP, VP, ClPO, and aryl oxidases. Which enzymes are involved in releasing specific types of lignin fragments? Do enzymes function synergistically to breakdown lignin? These are some of the questions that interest us. Bio-mimetic oxidation is carried out by activating hydrogen peroxide using Fe and Mn catalysts to oxidize a wide range of model lignin substrates and organosolv lignin. Our aim is to produce aromatic fragments of lignin, as well as to elucidate the mechanistic details of these processes. We are part of the Censurf Team, working with Peter C. Ford, Trevor W. Hayton, R. Daniel Little, Michelle A. O'Malley, Susannah Scott and Petra van Koppen at UCSB and our CenSURF colleagues at Washington University in St. Louis (Kevin D. Moeller), UC Davis (Louise Berben), UCSD (Clifford P. Kubiak), and Indiana University (Mu-Hyun Baik).