Research Projects
Synthetic Materials
POSS - PMA Oligomers
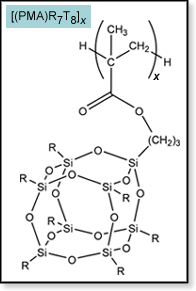 One of the promising systems for POSS-polymer formation begins with the n-propyl methacrylate ligand attached to an R7T8 POSS cage. This monomer has been successfully used to make homopolymers (see figure at right) as well as cross-linked copolymers. The [(PMA)R7T8]x polymers are amorphous, brittle plastics with high thermal stabilities. However, a total lack of structural information prevents any real understanding of the role POSS plays in modifying the properties of standard poly(n-propyl methacrylate). We are now using our technique of ion mobility mass spectrometry combined with molecular modeling to structurally characterize small PMA-POSS oligomers. A summary of our results so far is given below. Details can be found in "Structure of Hybrid Polyhedral Oligomeric Silsesquioxane Propyl Methacrylate Oligomers Using Ion Mobility Mass Spectrometry and Molecular Mechanics," S. E. Anderson, E. S. Baker, C. Mitchell, T. S. Haddad, M. T. Bowers, Chem. Mater. 2005, 17, 2537-2545
One of the promising systems for POSS-polymer formation begins with the n-propyl methacrylate ligand attached to an R7T8 POSS cage. This monomer has been successfully used to make homopolymers (see figure at right) as well as cross-linked copolymers. The [(PMA)R7T8]x polymers are amorphous, brittle plastics with high thermal stabilities. However, a total lack of structural information prevents any real understanding of the role POSS plays in modifying the properties of standard poly(n-propyl methacrylate). We are now using our technique of ion mobility mass spectrometry combined with molecular modeling to structurally characterize small PMA-POSS oligomers. A summary of our results so far is given below. Details can be found in "Structure of Hybrid Polyhedral Oligomeric Silsesquioxane Propyl Methacrylate Oligomers Using Ion Mobility Mass Spectrometry and Molecular Mechanics," S. E. Anderson, E. S. Baker, C. Mitchell, T. S. Haddad, M. T. Bowers, Chem. Mater. 2005, 17, 2537-2545
A sample of low-molecular-weight PMA-POSS oligomers was provided to us by our collaborators at Edwards AFB. A mass spectrum for the sample, shown below, was obtained on our MALDI-TOF instrument (oligomers ionized by sodium). Clearly the unreacted monomer left over from the polymerization process is the primary species observed but appreciable amounts of the dimer and trimer were also seen. We were able to detect species up to x = 8 in this sample but with insufficient intensity to obtain ATDs. The ATDs for the monomer, dimer and trimer are shown in the figure along with the cross section values obtained from them. 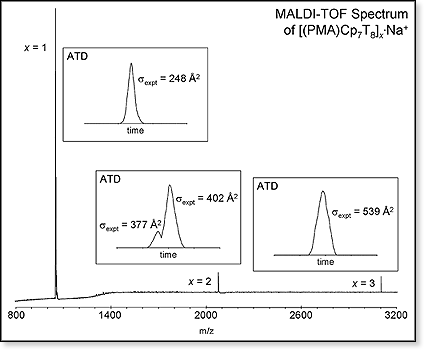 Both the monomer and trimer have narrow ATDs suggesting a single conformer/isomer family is involved for each system but the ATD for the dimer is bimodal, indicating the presence of at least two conformer families that do not interconvert on the millisecond timescale.
Both the monomer and trimer have narrow ATDs suggesting a single conformer/isomer family is involved for each system but the ATD for the dimer is bimodal, indicating the presence of at least two conformer families that do not interconvert on the millisecond timescale.
These three sodiated species were modeled with molecular mechanics to obtain a set of representative structures. For the monomer, only a single low-energy family of structures was observed with all structures having energies lying within 2.5 kcal/mol of each other. The average cross section of these structures was 251 Å2, in good agreement with experiment (248 Å2). The Na+ ion coordinates with four oxygen atoms on a face of the POSS cage and with one or two oxygen atoms on the PMA side chain.
| Theory | Experiment | ||||
|---|---|---|---|---|---|
a Values determined for structures in lowest 5 kcal/mol. |
|||||
| Structure | Cross Sect. | Abd. | Cross Sect. | Abd. | |
| Trans |
 |
377 | 24 | 378 | 20 |
| Extended Trans |
 |
393 | 46 | 402 | 80 |
| Cis |
 |
394 | 30 | ||
The PMA-POSS dimer has a bimodal ATD indicating at least two types of conformers are involved. Extensive modeling revealed three distinct families of structures distinguished by the way the POSS cages are linked to the backbone and oriented relative to each other. There are two somewhat extended families of structures and one more compact family of structures. The results are summarized in the table (right) where schematic structures are used to exemplify the POSS connectivities/orientations for the three families.
Extended dynamics calculations at 300 and 500 K indicate no interconversion between the structure types occurs. At 800 K the "trans" structure retains integrity but the "extended trans" and "cis" structures interconvert. All three structures have comparable energies according to modeling. However the modeling indicates 76% have the larger "extended trans" and "cis" conformations and 24% the more compact "trans" conformation. This result is in reasonable agreement with experiment where the peak with the longer arrival time in the ATD (larger cross section) is 80% of the total intensity and the peak with the shorter arrival time (smaller cross section) is 20%. Presumably the explanation for the result is entropic rather than energetic as there are simply more ways to configure the more extended species.
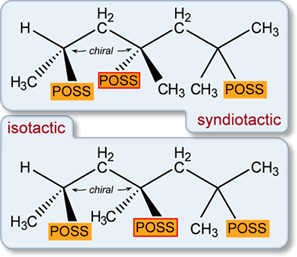 The trimer gives an ATD with a single peak. When three or more POSS cages are connected to the polymer backbone, the tacticity of the connections becomes an issue. The three common possibilities are isotactic (all POSS cages on the same “side”), syndiotactic (alternating POSS cage connections) and atactic (random connections). For the trimer, only the isotactic and syndiotactic isomers (shown schematically in the figure at left) are relevant. Molecular modeling was carried out for both isomers. In each case, the lowest-energy structures were the most compact but the syndiotactic isomer cross section agrees best with the experimental cross section: σexpt = 539 Å2, σsyn = 540 Å2 and σiso = 555 Å2. The model cross sections represent an average of the cross section of all structures in the lowest 5 kcal/mol of relative energy.
The trimer gives an ATD with a single peak. When three or more POSS cages are connected to the polymer backbone, the tacticity of the connections becomes an issue. The three common possibilities are isotactic (all POSS cages on the same “side”), syndiotactic (alternating POSS cage connections) and atactic (random connections). For the trimer, only the isotactic and syndiotactic isomers (shown schematically in the figure at left) are relevant. Molecular modeling was carried out for both isomers. In each case, the lowest-energy structures were the most compact but the syndiotactic isomer cross section agrees best with the experimental cross section: σexpt = 539 Å2, σsyn = 540 Å2 and σiso = 555 Å2. The model cross sections represent an average of the cross section of all structures in the lowest 5 kcal/mol of relative energy.
This is an interesting result. The two possible isomer families (syndiotactic and isotactic) have lowest-energy structures whose cross sections differ by 3%. The experimental ATD is narrow and shows only a single feature even when cooled to 80 K. If both isomers were present, a shoulder should have been observed. The conclusion is that only one of the isomers is being formed. While this is not a small system (over 560 atoms) we do believe our modeling protocols are still relatively accurate because of the degree of conservation of structure of the three Cp7T8 cages. Hence, we conclude that the syndiotactic isomer is dominantly and possibly exclusively made in the synthesis. This kind of specificity in reactions of this complexity is unusual and it will be important if we can show it continues for larger oligomers.
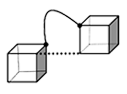
![[(PMA)Cp7T8]3](trimer.gif) A typical structure from the lowest-energy family of the syndiotactic isomers is shown at right (hydrogens and Cp groups omitted for clarity). The POSS cages are arranged in a near-equilateral triangle with a center-to-center distance in the range of 8.3 to 8.8 Å, very close to the "minimum" distance observed for the disiloxane, despite the much longer chain connecting the cages. This result supports the idea that the POSS cages tend to attract each other, at least when capped by Cp and attached with polar PMA linkers. Should this turn out to be a general result for the larger oligomers it will play an important role in understanding POSS-oligomer packing.
A typical structure from the lowest-energy family of the syndiotactic isomers is shown at right (hydrogens and Cp groups omitted for clarity). The POSS cages are arranged in a near-equilateral triangle with a center-to-center distance in the range of 8.3 to 8.8 Å, very close to the "minimum" distance observed for the disiloxane, despite the much longer chain connecting the cages. This result supports the idea that the POSS cages tend to attract each other, at least when capped by Cp and attached with polar PMA linkers. Should this turn out to be a general result for the larger oligomers it will play an important role in understanding POSS-oligomer packing.
The Na+ cation, required for detection of the oligomers, appears to play no role in determining the structure of the trimer. Extensive calculations indicate identical low-energy families of structures are obtained with and without the cation. This is an important point since it indicates our structural results accurately portray situations where cationizing agents are not involved.
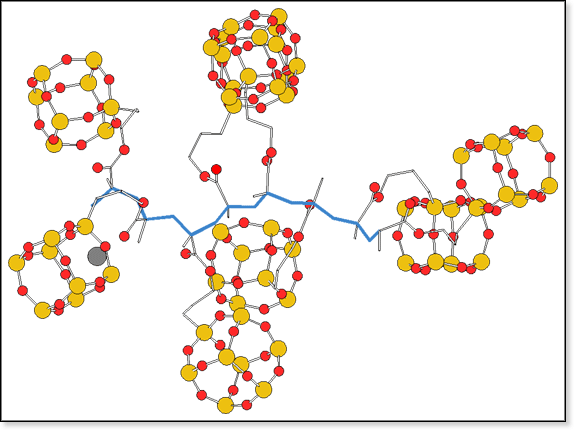
We plan on extending our investigations to higher-order PMA-POSS oligomers. As stated above, we have encountered signal intensity problems for the larger oligomers, but we hope to overcome these eventually. Despite the experimental challenges, we have already begun the theoretical calculations. The figure below shows a typical theoretical structure obtained for the PMA-POSS octamer [(PMA)i-Bu7T8]8·Li+ (hydrogens and capping groups omitted for clarity). As was seen in the [(PMA)Cp7T8]3·Na+ trimer above, the POSS cages tend to cluster together. The molecular mechanics calculations indicate that this is a result of van der Waals nonbonding interactions of the capping groups. As can be seen in the figure below, the metal ion coordinates with a single POSS cage and only one or two carbonyl groups. As a result, the oligomer backbone, highlighted in blue, is fairly extended. This contrasts with our earlier findings for poly(methyl methacrylate) that show the metal ion coordinates to multiple carbonyl oxygens with the backbone adopting a "U" shape. Thus, while the standard polymer bends to wrap around the ion, the hybrid polymer is apparently prohibited from doing so by the bulky POSS cages.