|
Introduction
The
ion mobility of a gas-phase ion is the quantity that describes how quickly
the ion moves through a drift cell filled with a high pressure of buffer
gas under the influence of a weak electric field. Small, compact ions
with small collision cross sections drift more quickly than large, extended
ions with large collision cross sections. This is similar to the effect
that causes an extended paper towel to drift to the ground much more slowly
under the influence of gravity and air resistance than a crushed towel
of the same mass. Thus, measuring the mobility of an ion yields information
about its structure, whether it is compact or extended. Mixtures of compact
and extended structures can be separated like in a chromatography experiment (hence "ion chromatography"). However, structural assignments based on
mobility results generally require comparison with calculated candidate
structures. Those are obtained in our lab by ab initio/density functional
theory calculations or molecular modeling using the AMBER force field.
(hence "ion chromatography"). However, structural assignments based on
mobility results generally require comparison with calculated candidate
structures. Those are obtained in our lab by ab initio/density functional
theory calculations or molecular modeling using the AMBER force field.
A
good reference for studying ion mobility theory is: Mason, E. A.; McDaniel,
E. W. Transport Properties of Ions in Gases; Wiley: New York, 1988.
The
key instrumental component required to measure an ion's mobility is the
drift cell. The details of this component vary depending on the instrument,
but in general it is made of copper or glass and has a drift length L
of 4-20 cm. It is typically filled with several Torr of helium and the
cell temperature can be varied from 80 K to more than 500 K. Guard rings
in the cell ensure a uniform electric field E which can be adjusted
from approximately 5 to 20 V/cm by changing the voltage V applied
across the cell.
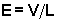
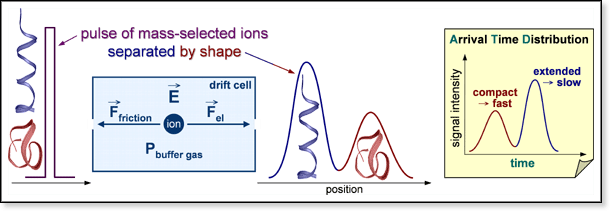
A pulse of
mass-selected ions is injected into the cell.* The force exerted on them
by the electric field is offset by the force of friction caused by collisions
of the ions with the buffer gas. As a result, ions drift through the cell
with a constant velocity vd that is proportional to
the electric field.
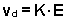
In this expression,
the proportionality constant K is called the ion mobility. The
ion mobility is directly proportional to the buffer gas pressure. It is
typically converted to a "reduced" mobility K0 using
a value of 760 Torr for P0 and 273.15 K for T0.
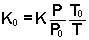
By combining
the equations above, an expression for the amount of time the ions spend
in the drift cell td can be obtained in terms of P/V.
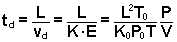
 The
quantity measured experimentally is ion intensity as a function of time,
generating a plot called an arrival time distribution (ATD). Arrival time
ta is the amount of time between the formation of the
initial ion pulse and an ion's arrival at the detector. It is equal to
td + t0 where t0
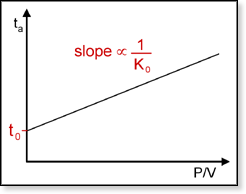
is the amount of time the ions spend outside the drift cell. Plotting
ta versus P/V for a given temperature yields
a straight line with an intercept equal to t0. The drift
time td can be obtained by subtracting t0
from ta. Typical drift times can range anywhere from
100 μs to 10 ms depending on the mobility of
the ion and the drift voltage used. The reduced mobility K0
can be obtained from the slope of the line. The Bowers Group uses this
type of experiments in several ways (see table below). In general, they
involve using the mobility to calculate a collision
cross section or using drift times to determine reaction
rate constants. The
quantity measured experimentally is ion intensity as a function of time,
generating a plot called an arrival time distribution (ATD). Arrival time
ta is the amount of time between the formation of the
initial ion pulse and an ion's arrival at the detector. It is equal to
td + t0 where t0
is the amount of time the ions spend outside the drift cell. Plotting
ta versus P/V for a given temperature yields
a straight line with an intercept equal to t0. The drift
time td can be obtained by subtracting t0
from ta. Typical drift times can range anywhere from
100 μs to 10 ms depending on the mobility of
the ion and the drift voltage used. The reduced mobility K0
can be obtained from the slope of the line. The Bowers Group uses this
type of experiments in several ways (see table below). In general, they
involve using the mobility to calculate a collision
cross section or using drift times to determine reaction
rate constants.

* All the
instruments in the Bowers group have mass-selection capabilities before
and after the drift cell except for the ESI
instrument, which only has it after the cell.
|

