Research Projects
Proteins
Aβ - Structural Details of the Monomer
We examine a range of Aβ peptides of various lengths and with various point mutations and look for specific structural elements such as loops, turns, and short sections of helix initiated by a specific amino acid sequence. A current working hypothesis is that certain structural elements of the monomer nucleate protein folding which in turn facilitates aggregation. Of particular interest are the sequence A21EDVGSNKGA30 and the hydrophobic C-terminal tail G29AIIGLMVGGVVIA42 which, in the full-length protein Aβ(1-42) and as fragments Aβ(21-30) and Aβ(29-42), are resistant to limited proteolysis. Our structural studies have focused on the peptides
- Aβ(1-42)
- Aβ(21-30)
- C-terminal fragments Aβ(x-42) and Aβ(x-40), x = 29 to 39
with wild-type sequences and sequences with selected point mutations.
-
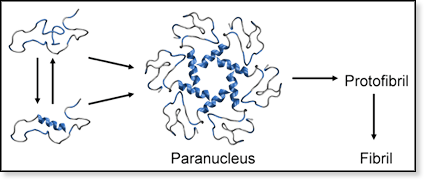 Figure 4. Schematic representation of Aβ42 conformations in the early stages of aggregation. Monomers without helical structure are in equilibrium with monomers containing small amounts of helical structure at the C-terminus. Five or six monomers assemble to form a paranucleus where the hydrophobic C-termini are shielded by the more hydrophilic parts of the peptides. Hydrophobic residues are marked in blue.
Figure 4. Schematic representation of Aβ42 conformations in the early stages of aggregation. Monomers without helical structure are in equilibrium with monomers containing small amounts of helical structure at the C-terminus. Five or six monomers assemble to form a paranucleus where the hydrophobic C-termini are shielded by the more hydrophilic parts of the peptides. Hydrophobic residues are marked in blue.
See also:
-
 Our study: Amyloid β-protein monomer structure: A computational and experimental study [3]
Our study: Amyloid β-protein monomer structure: A computational and experimental study [3]
-
 Our study: Structure of the 21-30 fragment of amyloid β-protein [4]
Our study: Structure of the 21-30 fragment of amyloid β-protein [4]
-
 Our web page: Peptide Folding
Our web page: Peptide Folding

