|
Peptide
Folding
 Peptides
are typically very flexible and have therefore a very large number of
possible conformations. Most of these conformations are energetically
unfavorable, but most peptides still have a number of conformations with
energies low enough to be populated under thermal conditions. For instance,
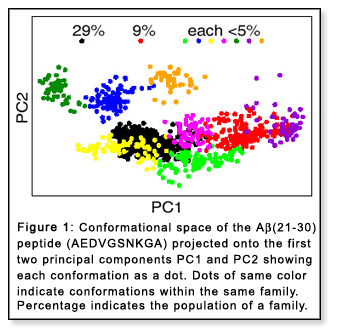
molecular mechanics calculations for the decapeptide Aβ(21-30)
indicate that hundreds of structures (which can be grouped into nine families)
are populated at room temperature (Figure 1).[1] Peptides
are typically very flexible and have therefore a very large number of
possible conformations. Most of these conformations are energetically
unfavorable, but most peptides still have a number of conformations with
energies low enough to be populated under thermal conditions. For instance,
molecular mechanics calculations for the decapeptide Aβ(21-30)
indicate that hundreds of structures (which can be grouped into nine families)
are populated at room temperature (Figure 1).[1]
However, for peptides with a charge-carrying unit
(e.g. a metal ion or a protonated group) all low-energy conformations
have a common structural motif in the absence of solvent: the charge is
buried in the interior of the molecule, well self-solvated (Figure 2).[2-6]
Hence, small charged peptides assume generally globular shapes. Globular
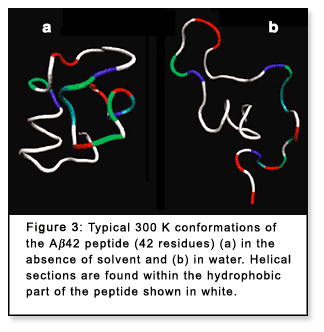
shapes are also observed for peptides with one or several salt bridges
even when the peptides are large (Figure 3a).[7] Hence,
in the absence of solvent the peptide shape is largely determined by maximizing
intramolecular electrostatic interactions which generally leads to compact
globular conformations. For peptid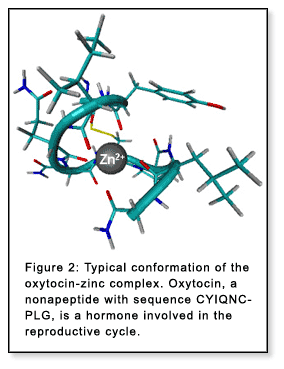 es
with a large content of hydrophobic residues, electrostatic interactions
are less significant. Consequently, the conformation of those peptides
can be determined by other factors, such as minimizing steric strain and
maximizing the number of hydrogen bonds in the system. Hence, hydrophobic
parts of peptides are found to form secondary structure such as helices
(Figure 3a). In extreme cases such as for polyalanines, formation of a
long helix can lead to very extended structures.[8] es
with a large content of hydrophobic residues, electrostatic interactions
are less significant. Consequently, the conformation of those peptides
can be determined by other factors, such as minimizing steric strain and
maximizing the number of hydrogen bonds in the system. Hence, hydrophobic
parts of peptides are found to form secondary structure such as helices
(Figure 3a). In extreme cases such as for polyalanines, formation of a
long helix can lead to very extended structures.[8]
Conformations of hydrated peptides, on the other
hand, are often much less compact than the low-energy gas-phase conformations
(Figure 3b).[7] In aqueous solution electrostatic
interactions are still important, but since there are both intramolecular
and water-solute intermolecular contributions, a poorly optimized intramolecular
electrostatic interaction can be compensated for by strong intermolecular
interactions.
|
|
Projects:
References:
- "Structure of the 21-30 fragment of Alzheimer's amyloid protein:
A molecular dynamics study" A. Baumketner, S. L. Bernstein, T.
Wyttenbach, N. D. Lazo, D. B. Teplow, M. T. Bowers, J.-E. Shea, manuscript
in preparation
- "Salt bridge structures in the absence of solvent? The case
for the oligoglycines" T. Wyttenbach, J. E. Bushnell, M. T. Bowers
J.
Am. Chem. Soc. 1998, 120, 5098-5103
- "Hydration of protonated primary amines: Effects of intermolecular
and intramolecular hydrogen bonds" D. Liu, T. Wyttenbach, M. T.
Bowers Int.
J. Mass Spectrom. 2004, 236, 81-90
- "The effect of the initial water of hydration on the energetics,
structures, and H/D exchange mechanism of a family of pentapeptides:
An experimental and theoretical study" T. Wyttenbach, B. Paizs,
P. Barran, L. Breci, D. Liu, S. Suhai, V. H. Wysocki, M. T. Bowers J.
Am. Chem. Soc. 2003, 125, 13768-13775
- "Gas-phase conformation of biological molecules: Bradykinin"
T. Wyttenbach, G. von Helden, M. T. Bowers J.
Am. Chem. Soc. 1996, 118, 8355-8364
- "Oxytocin-receptor binding: Why divalent metals are essential"
D. Liu, A. B. Seuthe, O. T. Ehrler, X. Zhang, T. Wyttenbach, J. F. Hsu,
M. T. Bowers J.
Am. Chem. Soc. 2005, 127, 2024-2025
- "Amyloid β-protein monomer structure: A computational and
experimental study" A. Baumketner, S. L. Bernstein, T. Wyttenbach,
G. Bitan, D. B. Teplow, M. T. Bowers, J.-E. Shea Protein Sci., accepted
for publication
- "Design of helices that are stable in vacuo" R. R. Hudgins,
M. A. Ratner, M. F. Jarrold J.
Am. Chem. Soc. 1998, 120, 12974-12975
|


