Research Projects
Proteins
Aβ - Overall Shape of Monomer & Small Oligomers
We evaluate the overall shape of monomers and small oligomers of wild-type Aβ in comparison with Aβ mutants that are known to form amyloid fibrils with different morphology and/or different kinetics than the wild type. The current hypothesis is that the shape of the tetramer determines whether oligomerization continues to hexamers – the paranuclei – and to dimers of hexamers.
-
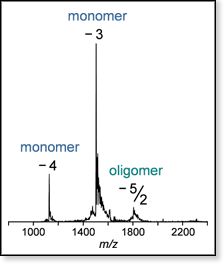 Figure 1. Mass spectrum of a sample of wild-type Aβ42 with peaks at the z/n-values noted (z is charge, n is oligomer size; n = 1 monomer, n = 2 dimer, etc.). The isotope patterns of the z/n = -3 and -4 peaks are consistent with monomer, whereas the isotope pattern of the z/n = -5/2 peak is not resolved, indicating the presence of oligomers larger than dimer.
Figure 1. Mass spectrum of a sample of wild-type Aβ42 with peaks at the z/n-values noted (z is charge, n is oligomer size; n = 1 monomer, n = 2 dimer, etc.). The isotope patterns of the z/n = -3 and -4 peaks are consistent with monomer, whereas the isotope pattern of the z/n = -5/2 peak is not resolved, indicating the presence of oligomers larger than dimer.
-
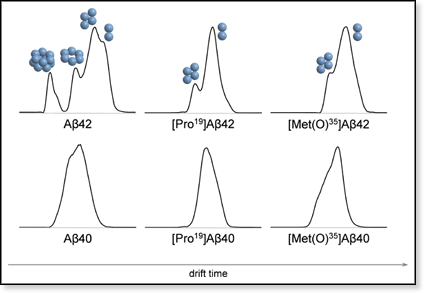 Figure 2. Ion mobility spectra for the z/n = –5/2 ions for samples of Aβ40, Aβ42, [Pro19]Aβ40, [Pro19]Aβ42, [Met(O)35]Aβ40, and [Met(O)35]Aβ42. All spectra look very similar (one major peak) except for wild-type Aβ42, which shows two baseline-resolved major features. The sequence of peaks and shoulders suggests the presence of dimer and tetramer in all six cases. However, for wild-type Aβ42 additional peaks and shoulders are apparent, which we assign as hexamer, decamer, and dodecamer. The gap between hexamer and decamer indicates complete absence of octamer, suggesting - together with the relatively high intensity of the dodecamer peak - formation of metaclusters, dimers of pentamers (Aβ5)2 and dimers of hexamers (Aβ6)2.
Figure 2. Ion mobility spectra for the z/n = –5/2 ions for samples of Aβ40, Aβ42, [Pro19]Aβ40, [Pro19]Aβ42, [Met(O)35]Aβ40, and [Met(O)35]Aβ42. All spectra look very similar (one major peak) except for wild-type Aβ42, which shows two baseline-resolved major features. The sequence of peaks and shoulders suggests the presence of dimer and tetramer in all six cases. However, for wild-type Aβ42 additional peaks and shoulders are apparent, which we assign as hexamer, decamer, and dodecamer. The gap between hexamer and decamer indicates complete absence of octamer, suggesting - together with the relatively high intensity of the dodecamer peak - formation of metaclusters, dimers of pentamers (Aβ5)2 and dimers of hexamers (Aβ6)2.
-
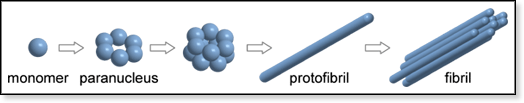 Figure 3. Proposed aggregation mechanism for Aβ42.
Figure 3. Proposed aggregation mechanism for Aβ42.
See also:
-
 Our study: Amyloid β-protein: Monomer structure and early aggregation states of Aβ42 and its Pro19 alloform [2]
Our study: Amyloid β-protein: Monomer structure and early aggregation states of Aβ42 and its Pro19 alloform [2]
-
 Our presentation: Significance of the tetramer structure (pp. 70-72) from a talk entitled Ion mobility: The method & recent applications given at the 55th ASMS Conference on Mass Spectrometry in June, 2007
Our presentation: Significance of the tetramer structure (pp. 70-72) from a talk entitled Ion mobility: The method & recent applications given at the 55th ASMS Conference on Mass Spectrometry in June, 2007

