Research Projects
Synthetic Materials
POSS - Monomers
| Formula | X-Ray | Ion Mob. (Na+)a | Theory (Na+)a |
|---|---|---|---|
a Cross sections include sodium ion (since this is what is observed in the ion mobility experiment) unless otherwise noted. b Calculated value for the neutral species. |
|||
| Cy6T6 | 224 | 221 | 222 |
| Ph8T8 | 260 | 257 | |
| Vi10T10 | 189 | 192 | |
| Vi12T12 | 212 | 214 | 216 |
| H14T14 | 137 | 139b | |
| Cy6T4D2(OH)2 | 222 | 220 | 222 |
| Cy7T4D2(OH)3 | 248 | 247 | 251 |
We have examined a variety of POSS monomers covering a range of cage sizes and capping groups.[1] Many of these species have been examined using x-ray crystallography by our synthetic collaborators at Edwards AFB. The table at right shows cross sections determined from the x-ray structure of a number of POSS monomers, including open-cage species (where D = SiO). ATDs for these species were also measured using both our ESI and MALDI-TOF instruments. Distributions with a single symmetric peak were obtained, indicating the presence of only one conformer for each compound. The corresponding cross sections are shown in the table for the ESI experiments. Very similar values were obtained using the MALDI-TOF instrument. Having x-ray structures of these species is essential in the molecular modeling aspect of our POSS studies. Our goal is to use ion mobility methods coupled with modeling to investigate POSS-polymers, compounds whose structures are difficult to obtain using the traditional methods of x-ray crystallography and NMR. The molecular mechanics force field that we use for modeling, AMBER, was not initially parameterized for silicon-containing molecules like POSS. The x-ray structures of POSS monomers provided a benchmark for the modeling and have allowed us to determine parameters for silicon. The cross sections associated with the theoretical structures are also given in the table. For each monomer, the cross sections determined by the three different methods all agree quite well.
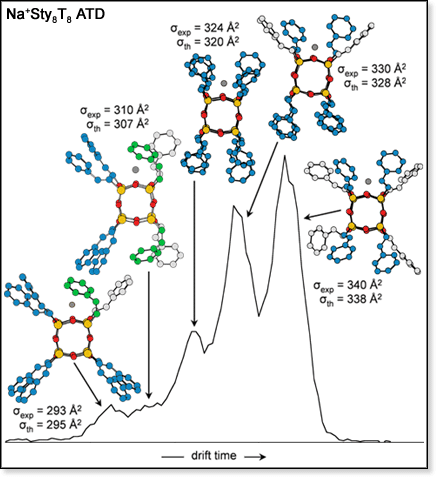 Another POSS monomer that we have investigated is Sty8T8, where the POSS cage capping agents are styryl groups (Sty).[2] Unlike species mentioned above, the ATD for the sodiated version of this monomer is not a single peak but has five resolvable features (the ATD, shown at left, was measured using our MALDI-TOF instrument). With theoretical modeling, we were able to identify the conformers giving rise to these features. Their structures are also shown in the figure (for clarity, hydrogen atoms are omitted). The three most intense peaks correspond to three low-energy families of conformers that differ in the number Sty groups that form interacting pairs (indicated by blue in the figure). The most extended of these three families occurs when only two pairs are formed. The conformers become more compact when three and four Sty pairs are formed. The relative intensities of these three peaks match the statistical expectation for pairing of nearest-neighbor capping groups. The remaining two smaller features in the ATD correspond to conformers even more compact than the one with four pairs of Sty groups. Up to this point in the modeling, it had been assumed that the styryl groups were rotated in such a way that the phenyl rings were "trans" to the Si-O cage, as indicated by the x-ray crystallography structure of Sty8T8. To account for the more compact conformers observed in the ATD, structures with one and two Sty groups rotated so that the phenyl ring is "cis" to the Si-O cage (as indicated by green in the figure) had to be considered. The resulting cross sections for these conformers were a good match for the experimental values. It is believed that these "cis-modifications" are impurities and occur during the synthesis of Sty8T8 rather than during the ion mobility experiments since changes in the ion mobility experimental conditions (specifically, injection energy) do not affect the ATD.
Another POSS monomer that we have investigated is Sty8T8, where the POSS cage capping agents are styryl groups (Sty).[2] Unlike species mentioned above, the ATD for the sodiated version of this monomer is not a single peak but has five resolvable features (the ATD, shown at left, was measured using our MALDI-TOF instrument). With theoretical modeling, we were able to identify the conformers giving rise to these features. Their structures are also shown in the figure (for clarity, hydrogen atoms are omitted). The three most intense peaks correspond to three low-energy families of conformers that differ in the number Sty groups that form interacting pairs (indicated by blue in the figure). The most extended of these three families occurs when only two pairs are formed. The conformers become more compact when three and four Sty pairs are formed. The relative intensities of these three peaks match the statistical expectation for pairing of nearest-neighbor capping groups. The remaining two smaller features in the ATD correspond to conformers even more compact than the one with four pairs of Sty groups. Up to this point in the modeling, it had been assumed that the styryl groups were rotated in such a way that the phenyl rings were "trans" to the Si-O cage, as indicated by the x-ray crystallography structure of Sty8T8. To account for the more compact conformers observed in the ATD, structures with one and two Sty groups rotated so that the phenyl ring is "cis" to the Si-O cage (as indicated by green in the figure) had to be considered. The resulting cross sections for these conformers were a good match for the experimental values. It is believed that these "cis-modifications" are impurities and occur during the synthesis of Sty8T8 rather than during the ion mobility experiments since changes in the ion mobility experimental conditions (specifically, injection energy) do not affect the ATD.
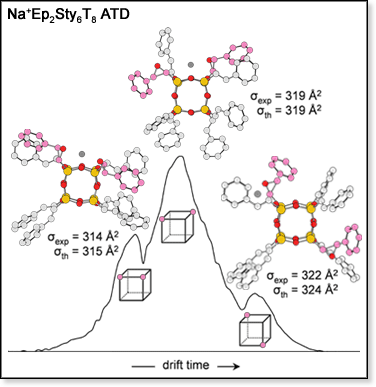 A group of related POSS species, EpxSty8-x, can be obtained by reacting Sty8T8 with epoxidizing agents. The EpxSty8-xT8 family is important in a number useful materials.[3] These species have Sty and epoxystyrl (Ep) capping groups, which allow the POSS cage to be networked into cross-linked polymers, such as epoxy and vinyl ester resins. ATDs for species with x = 1-3 were measured using our MALDI-TOF instrument. In each case a distribution with three peaks was observed. Using molecular modeling, we determined that the three features in the Na+EpSty7T8 ATD are due to pairings of the capping groups as was seen for Sty8T8 (above). For Na+Ep2Sty6T8 and Na+Ep3Sty5T8, geometric isomers give rise to the different peaks in the ATDs. The ATD for Na+Ep2Sty6T8 is shown in the figure at right, along with structures determined by molecular modeling (hydrogen atoms omitted). The most compact structure occurs when the two Ep groups (shown in pink) are on adjacent corners of the Si-O cage (as shown by the diagram used to label the corresponding ATD peak). The dominant feature in the ATD corresponds to the isomer in which the two Ep groups are diagonal to each other on one face of the Si-O cage. The most extended isomer has the two Ep groups on opposite corners of the Si-O cage and corresponds to the smallest feature in the ATD. Originally, the true composition of the EpxSty8-xT8 sample provided to us by our synthetic collaborators was unknown. Mass spectrometry and ion mobility techniques have allowed us to determine not only how many of the Sty groups were epoxidized to Ep groups during the synthesis, but where the Ep groups are located on the cages. This information, of course, is valuable if the EpxSty8-xT8 species are to be incorporated into polymer systems.
A group of related POSS species, EpxSty8-x, can be obtained by reacting Sty8T8 with epoxidizing agents. The EpxSty8-xT8 family is important in a number useful materials.[3] These species have Sty and epoxystyrl (Ep) capping groups, which allow the POSS cage to be networked into cross-linked polymers, such as epoxy and vinyl ester resins. ATDs for species with x = 1-3 were measured using our MALDI-TOF instrument. In each case a distribution with three peaks was observed. Using molecular modeling, we determined that the three features in the Na+EpSty7T8 ATD are due to pairings of the capping groups as was seen for Sty8T8 (above). For Na+Ep2Sty6T8 and Na+Ep3Sty5T8, geometric isomers give rise to the different peaks in the ATDs. The ATD for Na+Ep2Sty6T8 is shown in the figure at right, along with structures determined by molecular modeling (hydrogen atoms omitted). The most compact structure occurs when the two Ep groups (shown in pink) are on adjacent corners of the Si-O cage (as shown by the diagram used to label the corresponding ATD peak). The dominant feature in the ATD corresponds to the isomer in which the two Ep groups are diagonal to each other on one face of the Si-O cage. The most extended isomer has the two Ep groups on opposite corners of the Si-O cage and corresponds to the smallest feature in the ATD. Originally, the true composition of the EpxSty8-xT8 sample provided to us by our synthetic collaborators was unknown. Mass spectrometry and ion mobility techniques have allowed us to determine not only how many of the Sty groups were epoxidized to Ep groups during the synthesis, but where the Ep groups are located on the cages. This information, of course, is valuable if the EpxSty8-xT8 species are to be incorporated into polymer systems.
Our work on the POSS monomers also includes investigations of a variety of interesting species, including POSS-anilines, -imidophenyls and -norbornyls. We have also begun work on fluoride derivatives of POSS monomers. By incorporating fluoride ions, we hope to improve the ionization efficiency of our POSS samples and thereby overcome signal intensity problems we've encountered in systems that we would like to examine.
References
- "Application of Ion Mobility to the Gas-Phase Conformational Analysis of Polyhedral Oligomeric Silsesquioxanes (POSS)" J. Gidden, P. R. Kemper, E. Shammel, D. P. Fee, S. Anderson, M. T. Bowers Int. J. Mass Spectrom. 2003, 222, 63-73
- "3-Dimensional Structural Characterization of Cationized Polyhedral Oligomeric Silsesquioxanes (POSS) with Styryl and Phenylethyl Capping Agents" E. S. Baker, J. Gidden, D. P. Fee, P. R. Kemper, S. E. Anderson, M. T. Bowers Int. J. Mass Spectrom. 2003, 227, 205-216
- "Isomeric Structural Characterization of Polyhedral Oligomeric Silsesquioxanes (POSS) with Styryl and Epoxy Phenyl Capping Agents" E. S. Baker, J. Gidden, S. E. Anderson, T. S. Haddad, M. T. Bowers Nano Lett. 2004, 4, 779-785

