|
 Two
questions arise from the CID spectra of M+PMMAn: Two
questions arise from the CID spectra of M+PMMAn:
1. Why is the loss of
M+ more abundant for the larger alkali ions? (i.e. Why do the
bare Rb+ and Cs+ peaks dominate the CID spectra
while Na+ and Li+ do not?)
2. Why are there only
small m/z fragments in the CID spectra that originate from each end of
the oligomer? (i.e. Only A1, A2 and B1,
B2 fragments are observed. Why not A7 or B5?)
The
first question appears to be related to how well the PMMA oligomer can
wrap around the metal cation. The lowest-energy structures of the PMMA
9-mer cationized by Li+, K+, and Cs+
are shown at right. All three metal cations bind to carbonyl oxygens near
both ends of the oligomer, but the smaller Li+ ion is almost
enveloped by the PMMA oligomer while the larger K+ and Cs+
are significantly more exposed.
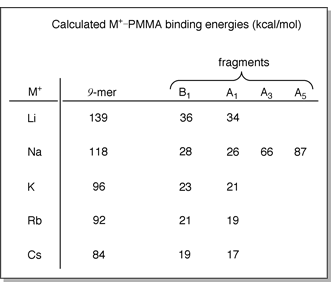
The larger metal cations also have significantly
smaller M+-PMMA binding energies (see table below left). Hence,
once energized by collisions with the bath gas in the CID experiments,
it is more energetically feasible to lose Cs+ than it is Li+.
The
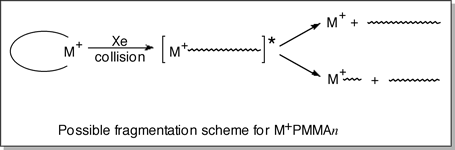
second question appears to be related to the U-shaped conformations that
the M+PMMAn oligomers fold into. Upon collisional activation,
the M+PMMA oligomer can simply "open up", leaving
M+ attached to either end of the oligomer. From this configuration,
the M+ ion can either dissociate (more likely for larger metal
cations) or induce a backbone cleavage nearby (generating Ax
and By fragments). This scheme is illustrated in the figure
below at right.
It
was originally proposed that the small Ax and By
fragments arose from sequential depolymerization of the PMMA oligomer
similar to poly(styrene) oligomers.
However, this would be an energetically uphill battle. Molecular mechanics
calculations on the formation of M+-Ax and M+-By
fragments indicate that M+ is bound much stronger to larger
A and B fragments. For example, the binding energy for Na+-A1
is 26 kcal/mol but the Na+-A5 binding energy is
87 kcal/mol.
Since only small m/z fragments (A1,
A2 and B1, B2) are observed in the CID
spectra, the M+ cations must preferentially induce cleavage
nearby. Also, once fragmentation occurs, the products must permanently
separate, otherwise the M+ cation would attach to the larger
neutral fragment (for energetic reasons) and larger Ax and
By fragments would be observed. This is possible if the M+PMMAn
oligomer is U-shaped. The initial "opening up" step would yield
a backbone that is highly excited. When a small fragment is cleaved, it
would have to leave at high velocity in order to conserve angular momentum.
|