 |

|
|||||
|
3-mer Dynamics - Li+PET3 |
|||||
 The
ATDs for Li+PET3 show
two peaks at all temperatures (80-550 K) corresponding to the "closed"
and "open" forms. Thus, the barrier that separates the two conformers
must be relatively high to prevent them from isomerizing at high temperatures
(see Na+PET3). The
ATDs for Li+PET3 show
two peaks at all temperatures (80-550 K) corresponding to the "closed"
and "open" forms. Thus, the barrier that separates the two conformers
must be relatively high to prevent them from isomerizing at high temperatures
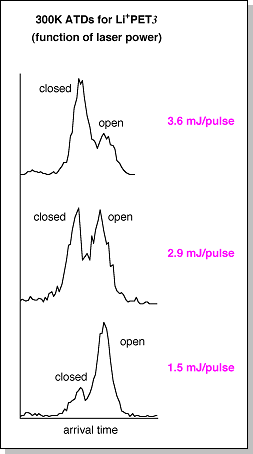
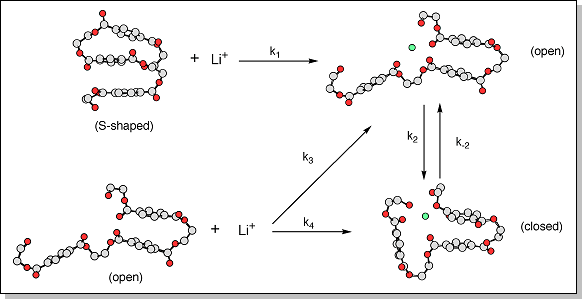
(see Na+PET3).Since the Li+PET3 ATDs are insensitive to temperature, the laser power used to desorb the ions in the MALDI source (see MALDI-sector instrument) was varied to determine whether the initial distribution of "closed" and "open" conformers could be changed. 300 K ATDs for Li+PET3 using three different laser power levels are shown at right. At the highest laser power, the "closed" form is more abundant but as the laser power is reduced the "open" form becomes favored. Theory, however, predicts that the "closed" form of Li+PET3 is ~5 kcal/mol lower in energy than the "open" form. So, why would a decrease in laser power, which decreases the energy available to the system, favor the higher-energy conformer? The reason may lie in how the Li+PET3 ions are formed. A possible mechanism that accounts for the features observed in the ATDs is shown below. Theory indicates that the lowest energy forms of neutral PET3 are S-shaped with the three phenyl groups stacked on top of each other. When this S-shaped oligomer encounters a lithium cation it must first fold into an "open" conformer (k1), as a "closed" conformer cannot be formed without proceeding through some type of "open" intermediate. Since the barrier between the "open" and "closed" forms of Li+PET3 is fairly large, the "open" form cannot easily convert into a "closed" form, especially at low laser powers (i.e. k2 is small). Thus, the "open" form is favored at low laser powers. If the laser power is increased, the Li+PET3 oligomers may have enough internal energy to overcome the isomerization barrier and the "open" forms can convert into the lower-energy "closed" forms. Additionally, at higher laser powers, excess vibrational energy may also induce the S-shaped neutral PET3 oligomers to open up. When this neutral open structure encounters a metal cation it can either form an "open" Li+PET3 oligomer (k3) or directly fold into a "closed" Li+PET3 oligomer (k4). Either way, the fraction of "closed" Li+PET3 oligomers increases only when the laser power is increased. The ATDs for Na+PET3 and K+PET3 were insensitive to the laser power. These oligomers also have smaller isomerization barriers than Li+PET3 so higher laser powers may not be needed to encourage the formation of the "closed" conformers. |
|||||
 |
|||||