|
Kinetics
- Direct Measurement
In
the direct-measurement method for investigating kinetics, ions are injected
into the drift cell where reactions takes place. For fragmentation reactions,
A+  B+
+ C, the drift cell contains an unreactive buffer gas and the fragmentation
kinetics are governed by the first order rate law. B+
+ C, the drift cell contains an unreactive buffer gas and the fragmentation
kinetics are governed by the first order rate law.
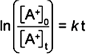
In
this expression, [A+]0 is the initial concentration
of the reactant ion A+ and [A+]t is the
concentration of A+ after a reaction time of t. The reaction
time is equal to the time the ions spend in the cell, i.e. the drift time.
The drift time, td, can be varied by changing the cell drift
voltage or the pressure of the gas in the cell and is determined by the
method described in the Ion Mobility Theory Introduction.
The concentration ratio is obtained from the mass spectrum of the ions
measured after they exit the drift cell. For the general reaction A+
 B+ + C,
the initial concentration of A+ is equal to the sum of the
concentrations of A+ and B+ at time t. The concentrations
are directly proportional to the intensities of the peaks in the mass
spectrum.* B+ + C,
the initial concentration of A+ is equal to the sum of the
concentrations of A+ and B+ at time t. The concentrations
are directly proportional to the intensities of the peaks in the mass
spectrum.*
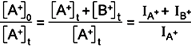
 In
this expression, IA+ and IB+
are the intensities of the A+ and B+ peaks in the
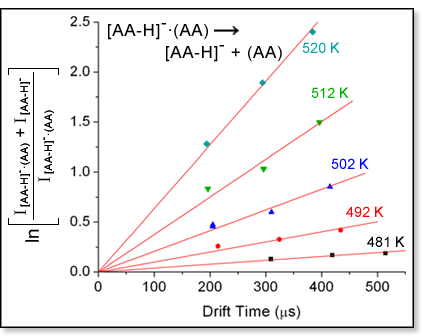
mass spectrum, respectively. A plot of the natural log of the peak intensity
ratio as a function of reaction time yields a straight line with a slope
equal to the rate constant k. The figure at left shows an example
of this type of plot at several temperatures for the dissociation of the
dialanine dimer [Liu,
D.; Wyttenbach, T.; Carpenter, C. J.; Bowers, M. T. J. Am. Chem. Soc.
2004, 126, 3261-3270]. By obtaining rate constants over
a range of temperatures, an Arrhenius analysis of the reaction can be
carried out. The Arrhenius equation indicates that plotting ln
k as a function of 1/T will yield a straight line with a
slope proportional to the activation energy Ea. In
this expression, IA+ and IB+
are the intensities of the A+ and B+ peaks in the
mass spectrum, respectively. A plot of the natural log of the peak intensity
ratio as a function of reaction time yields a straight line with a slope
equal to the rate constant k. The figure at left shows an example
of this type of plot at several temperatures for the dissociation of the
dialanine dimer [Liu,
D.; Wyttenbach, T.; Carpenter, C. J.; Bowers, M. T. J. Am. Chem. Soc.
2004, 126, 3261-3270]. By obtaining rate constants over
a range of temperatures, an Arrhenius analysis of the reaction can be
carried out. The Arrhenius equation indicates that plotting ln
k as a function of 1/T will yield a straight line with a
slope proportional to the activation energy Ea.
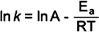
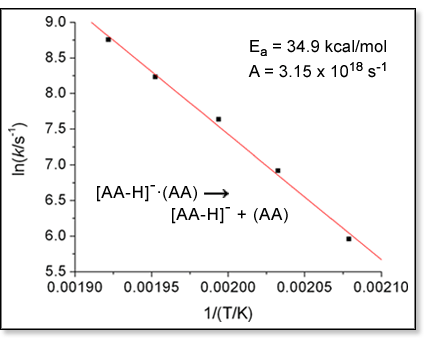 The
preexponential factor A, which is related to the entropy of activation
for the reaction, can be obtained from the y-intercept of the line. An
example of an Arrhenius plot is shown at right for the peptide dimer dissociation
reaction. The
preexponential factor A, which is related to the entropy of activation
for the reaction, can be obtained from the y-intercept of the line. An
example of an Arrhenius plot is shown at right for the peptide dimer dissociation
reaction.
The
direct-measurement kinetics method has also been used to determine rate
constants for bimolecular reactions with multiple product channels, A+
+ B  productsi
[Manard, M. J.; Kemper, P. R.; Carpenter, C. J.; Bowers, M. T.
Int.
J. Mass Spectrom. 2005, 241, 99-108]. For these types of reactions, ions are injected into
the drift cell containing the neutral reactant. The reactions can be considered
pseudo-first order under our experimental conditions since the neutral
reactant B is present in much greater concentrations in the drift cell
than the ionic reactant A+. In these cases the plot generated
to determine the rate constants will have a slope that incorporates the
number density of the neutral reactant gas ρ(B). productsi
[Manard, M. J.; Kemper, P. R.; Carpenter, C. J.; Bowers, M. T.
Int.
J. Mass Spectrom. 2005, 241, 99-108]. For these types of reactions, ions are injected into
the drift cell containing the neutral reactant. The reactions can be considered
pseudo-first order under our experimental conditions since the neutral
reactant B is present in much greater concentrations in the drift cell
than the ionic reactant A+. In these cases the plot generated
to determine the rate constants will have a slope that incorporates the
number density of the neutral reactant gas ρ(B).
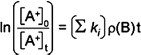
In this expression,
Σki is the sum of the rate constants for each
product channel.
* Note: This
assumes instrumentational mass discrimination is insignificant. Experience
has shown this to be the case in all of the systems we have studied.
|

