 |

|
|||||||||
|
Theoretical Structures of PEG, PPG, and PTMEG - obtained using molecular mechanics methods |
|||||||||
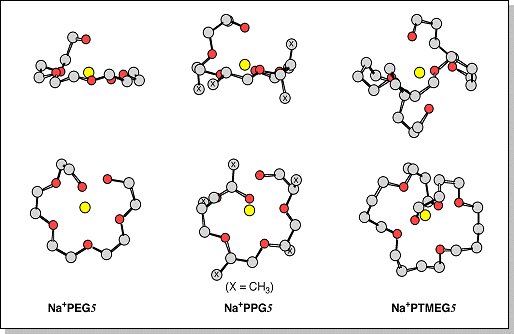
structures (two views, each) for the PEG, PPG, and PTMEG 5-mers cationized by Na+. Each polyether forms a ring around the Na+ ion with all 6 available oxygens coordinating to the metal cation. The planarity of the ring, however, is dependent on the oligomer. |
 |
||||||||
cationized by Na+. Oxygens that are < 3 Å away from Na+ are shown in purple. In each case, the arrangement of oxygens around the Na+ ion is similar to that predicted for the 5-mers. However, the Na+ ion coordinates to 8 oxygens in PEG but only 7 oxygens in PPG and 6 oxygens in PTMEG. 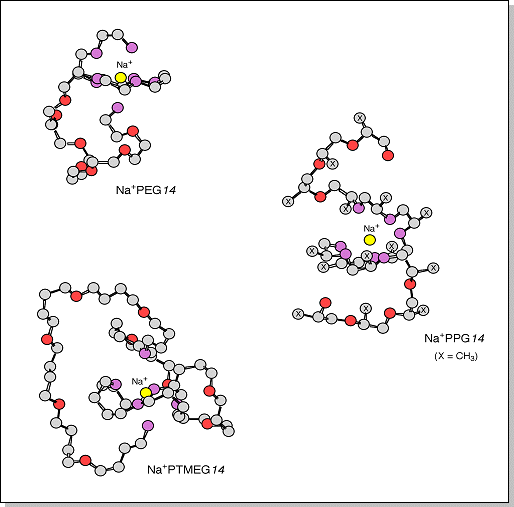 |
mer cationized by Li+ and Cs+. At small n, the oligomers must extend themselves to wrap around the larger metal cations such as Cs+. Therefore, the cross-sections for the smaller Cs+ oligomers are larger than the Li+ or Na+ oligomers. At larger n, the oligomers can efficiently wrap around larger metal cations and M+ becomes buried in the oligomer and the average cross-section of M+PPG and M+PTMEG becomes independent of M+. 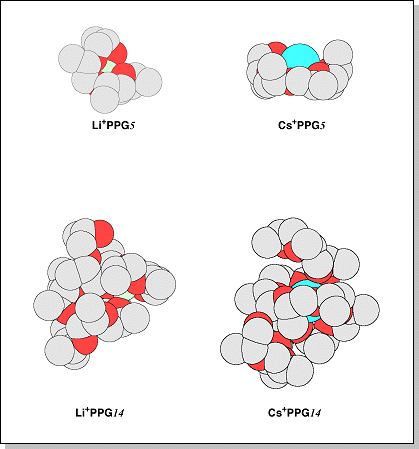
|
||||||||