
|
|
|
 |
|
 |
|
|
Zwitterions
- Effects on Stability
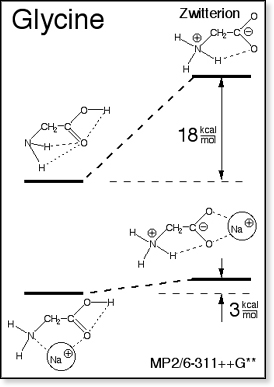 Zwitterions
such as the glycine zwitterion shown in the figure at right are intrinsically
(i.e. in the gas phase) not very stable. Solvation is a major contribution
to stabilizing zwitterions. For
instance, the glycine zwitterion has been calculated to be unstable by
~18 kcal/mol, whereas the zwitterion solvated by water molecules is more
stable than neutral glycine by ~11 kcal/mol.[3,4] Zwitterions
such as the glycine zwitterion shown in the figure at right are intrinsically
(i.e. in the gas phase) not very stable. Solvation is a major contribution
to stabilizing zwitterions. For
instance, the glycine zwitterion has been calculated to be unstable by
~18 kcal/mol, whereas the zwitterion solvated by water molecules is more
stable than neutral glycine by ~11 kcal/mol.[3,4]
The main effect of adding cations to a zwitterion
is the same as that of solvation: stabilization of the zwitterion with
respect to the neutral form. For instance, adding a sodium ion to the
glycine zwitterion makes the zwitterion almost as stable as the sodiated
glycine charge solvation structure. For small glycine-like systems the
amount of zwitterion stabilization depends strongly on the nature of the
cation.[6,7] Larger
alkali ions stabilize the zwitterion less than smaller alkali ions. However,
for glycine none of the alkali ions is able to bring down the energy of
the zwitterion below the level of the most stable charge solvation structure.
The alkali ion does not only affect the stability of the zwitterion, but
also the satiability of various charge solvation structures with respect
to each other. For the small alkali ions such as sodium the charge solvation
structure CS1 (see figure at left)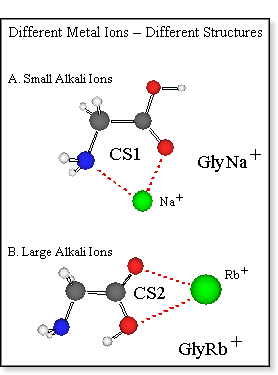 is the most stable structure, whereas CS2 is more stable for the larger
alkali ions such as rubidium.[7]
is the most stable structure, whereas CS2 is more stable for the larger
alkali ions such as rubidium.[7]
For glycine-like amino acids (points a-f in the
figure below) there is a nice nearly-linear relationship between zwitterion
stability (as calculated at the B3LYP/DZVP level)
and proton affinity (PA).[7]
However, for systems different from "glycine-like", other effects
factor in causing deviation from the linearity observed for points a-f.
This is evident for N-amidino-glycine, point g in the figure below. In
N-amidino-glycine the amino group present in glycine is replaced by a
guanidino group making it much more basic (increased PA) than glycine,
but causing structural changes as well.[8]
The effect most likely responsible for the deviation from the ΔE
vs. PA linearity is the fact that the electrical dipole in the N-amidino-glycine
zwitterion is different in orientation than in the glycine zwitterion.
|
|
|
 FIGURE
(right): Zwitterion (ZW) stability (as calculated at the B3LYP/DZVP level)
with respect to charge solvation stability (CS) of sodiated systems as
a function of amino acid proton affinity (PA). FIGURE
(right): Zwitterion (ZW) stability (as calculated at the B3LYP/DZVP level)
with respect to charge solvation stability (CS) of sodiated systems as
a function of amino acid proton affinity (PA).
(a) glycine, (b) alanine, (c) alpha-isobutyric acid
(d) sarcosine, (e) proline, (f) N,N-dimethyl-glycine
(g) N-amidino-glycine
|
|
|
 In
fact, in sodiated glycine-like zwitterions the zwitterion dipole is almost
ideally aligned with the sodium charge, making the salt bridge structure
unusually stable. In N-amidino-glycine the dipole is less favorably aligned
with the sodium charge as schematically shown in the figure to the left.
Hence, the zwitterion is less stable than expected on the basis of a comparison
with glycine-like systems and the very high PA value of N-amidino-glycine.
Poorer dipole-charge alignment is also present in salt bridge structures
of small peptides, an effect contributing to the generally lower stability
of cationized peptide zwitterions compared to cationized amino acids.
For instance, the sodiated diglycine zwitterion is 14 kcal/mol less stable
than the charge solvation structure, whereas the sodiated glycine zwitterion
is only 3 kcal/mol above the charge solvation structure.[5] In
fact, in sodiated glycine-like zwitterions the zwitterion dipole is almost
ideally aligned with the sodium charge, making the salt bridge structure
unusually stable. In N-amidino-glycine the dipole is less favorably aligned
with the sodium charge as schematically shown in the figure to the left.
Hence, the zwitterion is less stable than expected on the basis of a comparison
with glycine-like systems and the very high PA value of N-amidino-glycine.
Poorer dipole-charge alignment is also present in salt bridge structures
of small peptides, an effect contributing to the generally lower stability
of cationized peptide zwitterions compared to cationized amino acids.
For instance, the sodiated diglycine zwitterion is 14 kcal/mol less stable
than the charge solvation structure, whereas the sodiated glycine zwitterion
is only 3 kcal/mol above the charge solvation structure.[5]
For
cationized peptides, self-solvation becomes with increasing peptide chain
length an increasingly more important effect for stabilizing charge solvation
structures. This is evident for sodiated pentaglycine, where solvation
of the sodium ion is maximized with the presence of five solvating >C=O
groups (figure below, right).[5]
For hexaglycine, coordination of five >C=O groups (not six) is observed
as well.[5] (See molecular
modeling results confirmed by cross
section measurements.)  Hence,
for peptides larger than pentapeptides, charge solvation structures cannot
benefit much more by an additional increase of system size. However, complete
self-solvation of a zwitterion structure with three charges (2 positive,
1 negative) is only achieved for considerably larger peptides (possibly
as large as a 15-residue peptide, 5 residues per charge). Therefore, salt
bridge structures are expected to benefit from increasing system size
in the range from 5 to 15 residues. An example of a zwitterionic peptide
in this size range is probably protonated bradykinin.[14] Hence,
for peptides larger than pentapeptides, charge solvation structures cannot
benefit much more by an additional increase of system size. However, complete
self-solvation of a zwitterion structure with three charges (2 positive,
1 negative) is only achieved for considerably larger peptides (possibly
as large as a 15-residue peptide, 5 residues per charge). Therefore, salt
bridge structures are expected to benefit from increasing system size
in the range from 5 to 15 residues. An example of a zwitterionic peptide
in this size range is probably protonated bradykinin.[14]
|
|
|
Note
on B3LYP/DZVP:
Density
Functional Theory (DFT) calculation using exchange and correlation functionals
calculated by Becke’s Three Parameter Hybrid Method (Becke,
A. D. J. Chem. Phys. 1993, 98, 5648-5652) including
the LYP (Lee, Yang, Parr) expression (B3LYP) with a basis set of double-zeta
quality for the valence electrons plus polarization functions on heavy
atoms comparable to 6-31G* (Godbout, N.; Salahub, D. R.; Andzelm, J.;
Wimmer, E. Can. J. Chem. 1992, 70, 560-571).
|
|

